Liquid Crystal Polymers (LCPs)
Properties
Liquid crystalline polymers (LCPs) are a special type of
thermoplastics that exhibit properties between highly ordered solid
crystalline materials and amorphous disordered liquids over a well
defined temperature range.
To date, thousands of LC polymers have
been synthesized. However, only a small number have become
commercially important LC materials. The three most common LCPs are
semi-aromatic copolyesters, copolyamides, and polyester-co-amides.
These polymers contain rigid rod-like or plate-like repeat units
with a high length-to-width ratio, the so called mesogenic groups,
that are able to self-assemble into anisotropic liquid crystals
(mesophases) upon cooling or under the action of an external field.
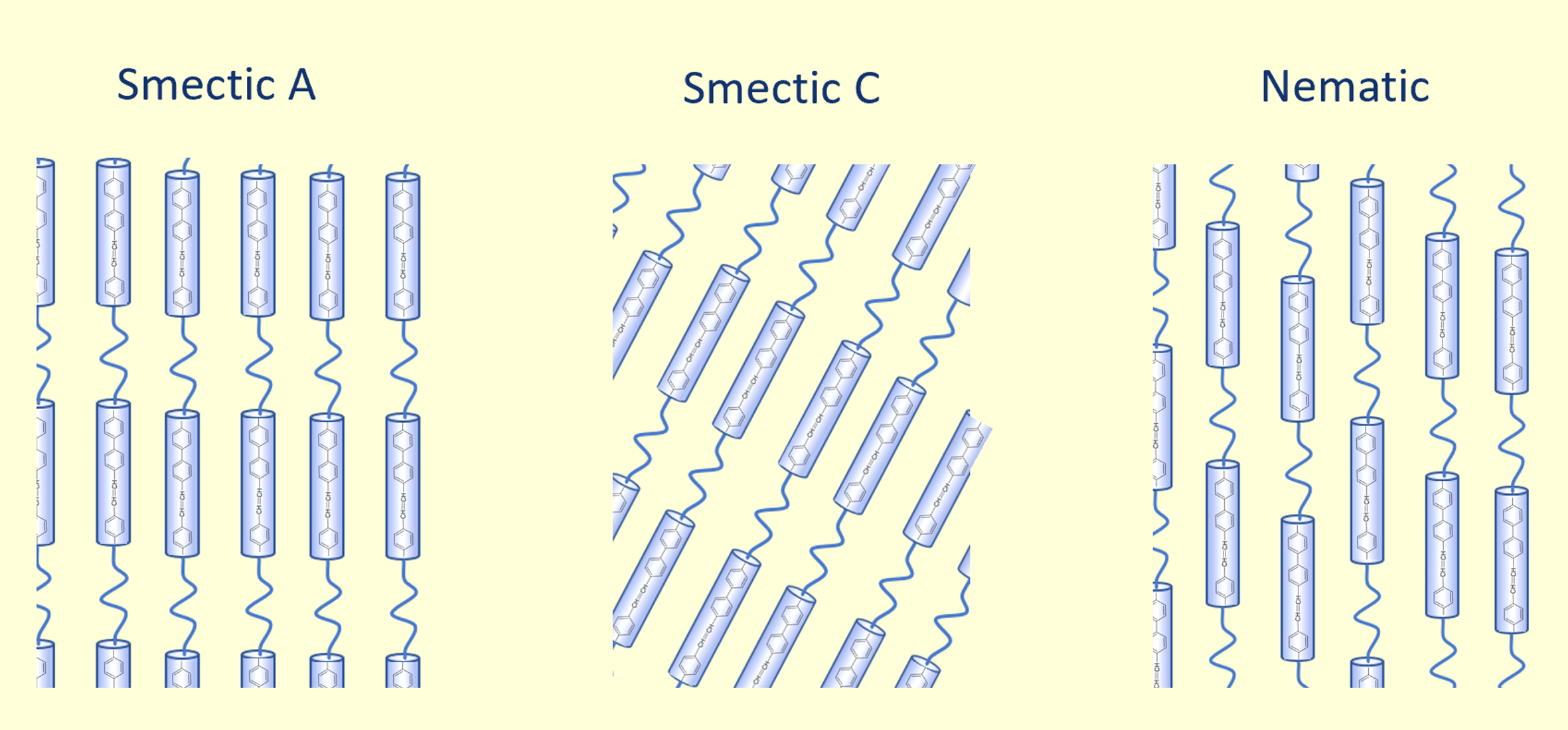
Three very common LC mesophases are nematic, smectic A and
smectic C
(see below). Nematic mesophases show only unidimensional orientational
order in the direction of the long (in rod-shaped) or short (in disc-shaped) molecular axes.
This is typically the flow direction during processing of the LC
resin. Smectic mesophases, on the other hand, show two-dimensional
orientational order. These LC’s have a lot in common with crystalline polymers where molecules are arranged in layers (lamellae).
The long axes of the (rod-like) molecules are either perpendicular to the plane (smectic A) or
it is inclined at an angle (smectic C).
Examples of Common Mesophases
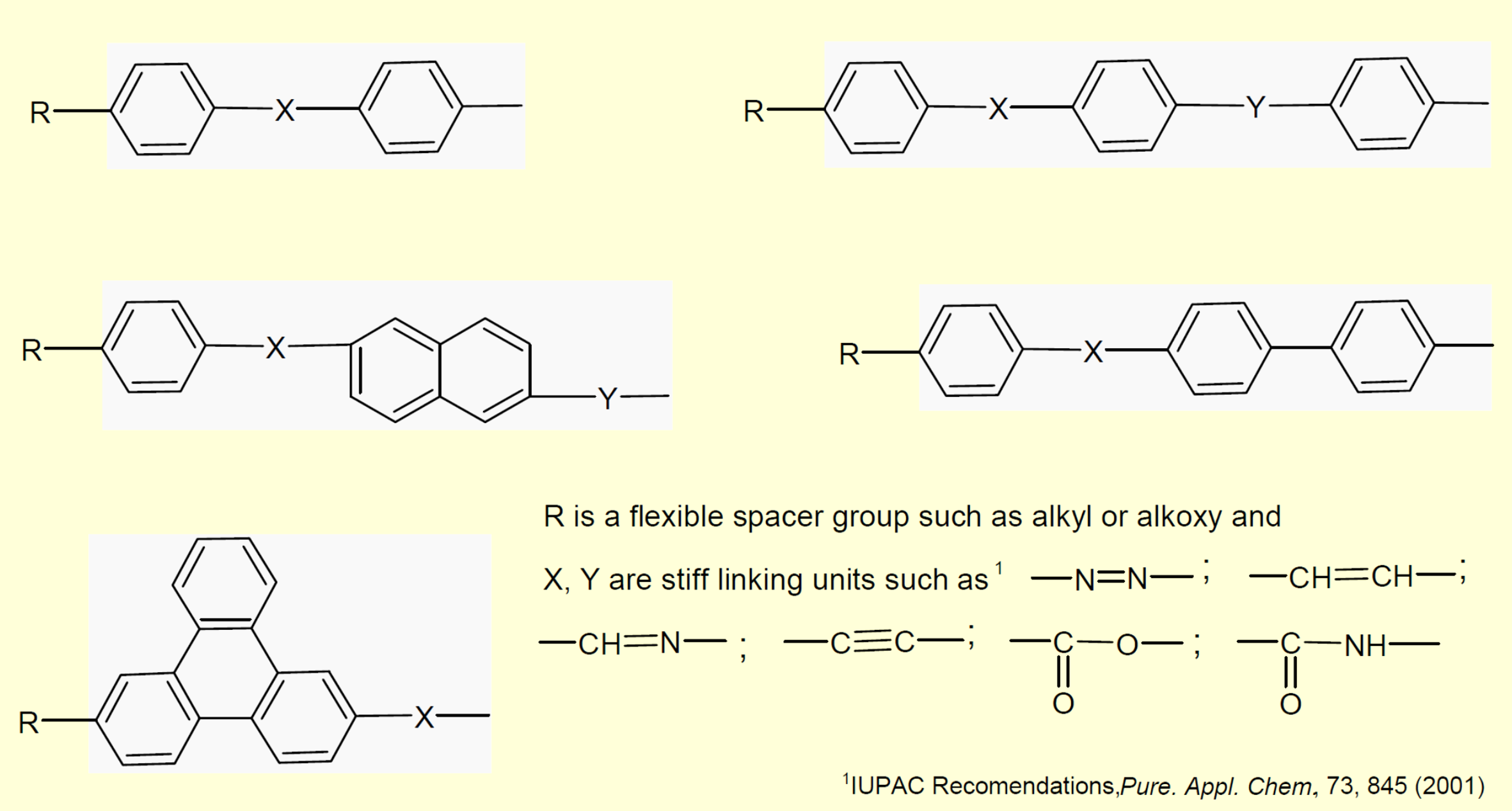
The most common LCPs are liquid crystalline polyesters which can be produced by polycondensation of an aromatic hydroxycarboxylic acid and an aromatic dicarboxylic acid with an alipahtic diol as a coreactant. The aromatic blocks form liquid crystals in the copolymer while the aliphatic blocks act as flexible spacers. Several other flexible (hydrophobic) spacers such as alipahtic ethers and organosilicons can be part of the LCP. The spacers or flexible linkers impart mobility to the rigid mesogenic groups1 to ease cooperative self-assembly. They also lower the melting point and increase the temperature range within which the liquid crystals are stable.
The properties of LCPs such as crystallinity, flexibility, and strength depend on the molecular weight distribution, the average chain length and the type and length of the flexible linker or spacer. The properties can be further adjusted by copolymerization with other monomers and by addition of plasticizers and fillers and/or by blending with other polymers such as polycarbonates, polyolefins, and polyesters. The addtion of plasticizers and fillers lowers cost, alters the performance and can facilitate processing. For example, addition of graphite improves resistance to chemicals and wear, whereas carbon fibers and glass fibers increase strength and rigidity; carbon black enhances electrical properties such as electrostatic dissipation.
Many commercial self-reinforced LCPs can be processed using conventional injection molding and extrusion techniques. For fiber applications, LCPs are spun into fibers from lyotropic solutions or melts.2 The extrusion of the liquid or dissolved LC polymers through spinnerets or small nozzles produces highly anisotropic plastic fibers that have very high tenacity and elastic modulus.
Modern plastics based on LC polymers are often referred to as superplastics because they possess much higher elastic modulus (up to 60-70 GPa) and strength (up to 700 MPa) than conventional plastics. They also exhibit very low elongation at break (typically in the range of 1.5 - 2%), high stiffness and very low thermal expansion coefficients (CTE) which are noticeably lower than those of conventional non-liquid crystalline polymers and often comparable with the CTEs of inorganic glasses.2 For example, the tenacity of aromatic LC polyamide fibers is typically 2 to 3 times higher and the elastic modulus is about 10 to 20 times higher than those of high-strength yarns based on aliphatic polyamides such as nylon 6,6.3 Aromatic polyamides have also a favorable strength-to-weight ratio due to their low density. For example, their specific strength is about 8 times higher than that of steel and comparable to those of carbon fibers and glass fibers.3
The enhanced mechanical properties of LCPs are the result of a more dense packing of the polymer chains in the LC phase due to the parallel alignment of the rigid mesogenic groups which is further enhanced by extrusion, spinning and drawing. The highly ordered structure and the resulting low void density present in LCPs is also responsible for the much lower gas permeability and exceptionally good hydrolytic and chemical resistance including many (diluted) acids / alkalines, and a large number of common liquids and solvents.4
The anisotropic LC structure intermediate between the crystalline solid and disordered liquid state is unique to liquid crystal polymers.5 In the liquid crystalline state, the ordered grouping of the rigid segments is sufficient to impart some solid-like behavior on the plastic, but the attractive forces between the aligned molecules are not strong enough to prevent some movement of the rigid crystalline domains when exposed to external forces or fields. The high mobility of the crystals allows for molecular rearrangement of the crystals and thus for manipulation of the anisotropic properties by mechanical stress, heat, radiation, electromagnetic fields, and in some cases, even by the chemical environment. This behavior makes LCPs very desirable as functional materials for a broad spectrum of optic and optoelectronic applications.
Examples of Typical Mesogenic Units
Commercial Liquid Crystal Polymers
The highest volume commercial LCPs are liquid crystalline
polyesters. One of the cheapest is
self-reinforcing polyester which can be produced by co-polycondensation of p-hydroxybenzoic acid and terephthalic acid
and/or 4,4i-dihydroxydiphenyl with ethylene glycol as a coreactant. Important manufacturers of
this type of LCP are Celanese and Solvay.
A common fully aromatic liquid crystalline polyester is based on copolyesters of p-hydroxybenzoic acid and 6-hydroxy-2-naphthoic acid which is sold under several trade names. Two important manufacturers of polyester-based LC polymers are Celanese and Kuraray which market these products under the trade names Vectran™, Vectra® and Zenite LCP ®. Various grades of these resins are available on the market including unreinforced, glass and carbon fiber reinforced, PTFE modified (Vectra) as well as mineral, graphite and carbon black loaded. Many of these resins can be easily molded with standard processing techniques including injection molding, extrusion, co-extrusion and thermoforming.
An important LC polymer based on aromatic amide is poly(p-phenylene terephthalamide) also known as Kevlar®. Three important manufacturers of aromatic polyamides are Honeywell (Spectra®), Teijin (Twaron®) and DuPont (Kevlar®). Commercially, these LC polymers are mainly sold in the form of fibers. The fibers are typically wet spun which involves extruding the dissolved resins through small orifices of spinnerets into an aqueous coagulation bath to extract the solvent. The fibers are subsequently drawn at high temperature to increase their stength.
Applications
LC polymers are much more expensive than standard thermoplastics but have excellent mechanical and electrical properties and outstanding chemical resistance. They are used for very demanding applications in many industries. Examples include ultra-high-strength and lightweight fibers and cables, bulletproof vests, tennis strings, hockey sticks (as a composite), snowboards, tire reinforcement, jet engine enclosures, brake and transmission friction parts, and gaskets.
LC polymers are also widely used as functional materials in all kinds of optic and optoelectronic devices because their anisotropic material properties such as refractive index, birefringence, selective light reflection and transmitance, color characteristics are tunable by temperature, mechanical stress, and electromagnetic radiation and fields. Important applications include temperature-sensing, data storage, display technology, telecomunication and numerous other optic and optoelectronic products.
1Mesogens or mesogenic groups are the parts of the polymer that self-assemble and phase-microsegregate into a number of ordered structures or domains. The rigid mesogens can be located in the main chain or in the side chains (branches) or they can be the polymer itself.
2V.P. Shibaev, A.Y. Bobrovsky, Russ. Chem. Rev., 86 (11) 1024-1072 (2017)
3Kevlar® Aramid Fiber Technical Guide, H-77848 4/00
4The chemical resistance depends on the chemical structure and composition of the LCP as well as on the test condition such as temperature and time of exposure.
5The liquid-crystalline state is only retained within a certain temperature range. More specifically, the LC state exists only between the glass transition temperature (or melting temperature, if a polymer is able to crystallize) and the isotropization temperature (Tiso). Above Tiso, the polymer loses its LC properties and behaves like an ordinary isotropic polymer melt whereas below its glass tranasition temperature (Tg) the polymer behaves like a (semi-)crystalline solid but with anisotropic properties because the anisotropic structure is fully preserved below the Tg.